February 16, 2023
Finding a more effective way to treat infantile hemangioma
Dr. Beth Drolet, chair of Dermatology at UW Health, believes her approach to treating these birthmarks may be safer and more effective for babies. A new business, Arkayli, hopes to bring this novel approach to market.
Parents of a new baby are typically beaming with joy, at least when they are not excessively sleep-deprived. Such joy, however, can suddenly turn to worry if the baby develops an unusually bright red mark or a purplish, birthmark on their face, arms or anywhere on the body. The clinical diagnosis for these birthmarks is called infantile hemangioma, or IH for short.
Unlike conventional birthmarks, infantile hemangiomas typically change in appearance, first by getting bigger and then gradually resolving over several years. Infantile hemangiomas are the most common benign tumor found during the first year of life. They are diagnosed in about 170,000 babies born in the US annually, or between 1 and 3 percent of births.
About half the babies diagnosed with IH develop complications, but it’s not always easy to distinguish the infants who need treatment from those who don’t. Because most serious hemangiomas grow quickly, babies can develop scars even if the visible mark disappears. In other cases, breathing or vision may be impaired if a protruding mark appears on the nose or near the eye.
Parents of these babies are understandably anxious. Pediatricians often refer the families to a pediatric dermatologist, but even the most experienced experts acknowledge that existing beta blocker treatments are not optimal because they are absorbed systemically, especially at such a young age.
“We’ve been using two different beta blockers, propranolol and timolol, for about the past 15 years,” says Dr. Beth Drolet, a UW Health pediatric dermatologist whose clinical practice and research has focused on IH for three decades. “These drugs are usually effective, but they’re not ideal,” she adds, “More commonly prescribed for adults with heart conditions or high blood pressure, beta blockers can present several unwanted side effects.”
In addition, Drolet adds, parents need to feed the baby before each dose (every 3–4 hours), which means waking the baby during the night.
Goal is to develop a topical approach
The optimal solution, Drolet says, would be to safely deliver the beta blocker directly to the skin without it getting into the bloodstream.
As chair of the Department of Dermatology at the UW School of Medicine and Public Health, Drolet hasn’t been just waiting around for something better. She has, in fact, been at the forefront of developing a treatment methodology that could realize her dream and make life for these children and families much easier.
Working with a Madison-based biopharma company called Arkayli, co-founded by Drolet, she and her Arkayli colleagues see great promise for a new treatment, known as ARK001.
“The idea is fairly simple,” Drolet says. “Instead of giving the baby a liquid by mouth, parents can apply the formulation directly on the surface of the skin. The goal is to provide targeted treatment quickly and safely, before the hemangioma has a chance to cause bigger health or cosmetic problems.”
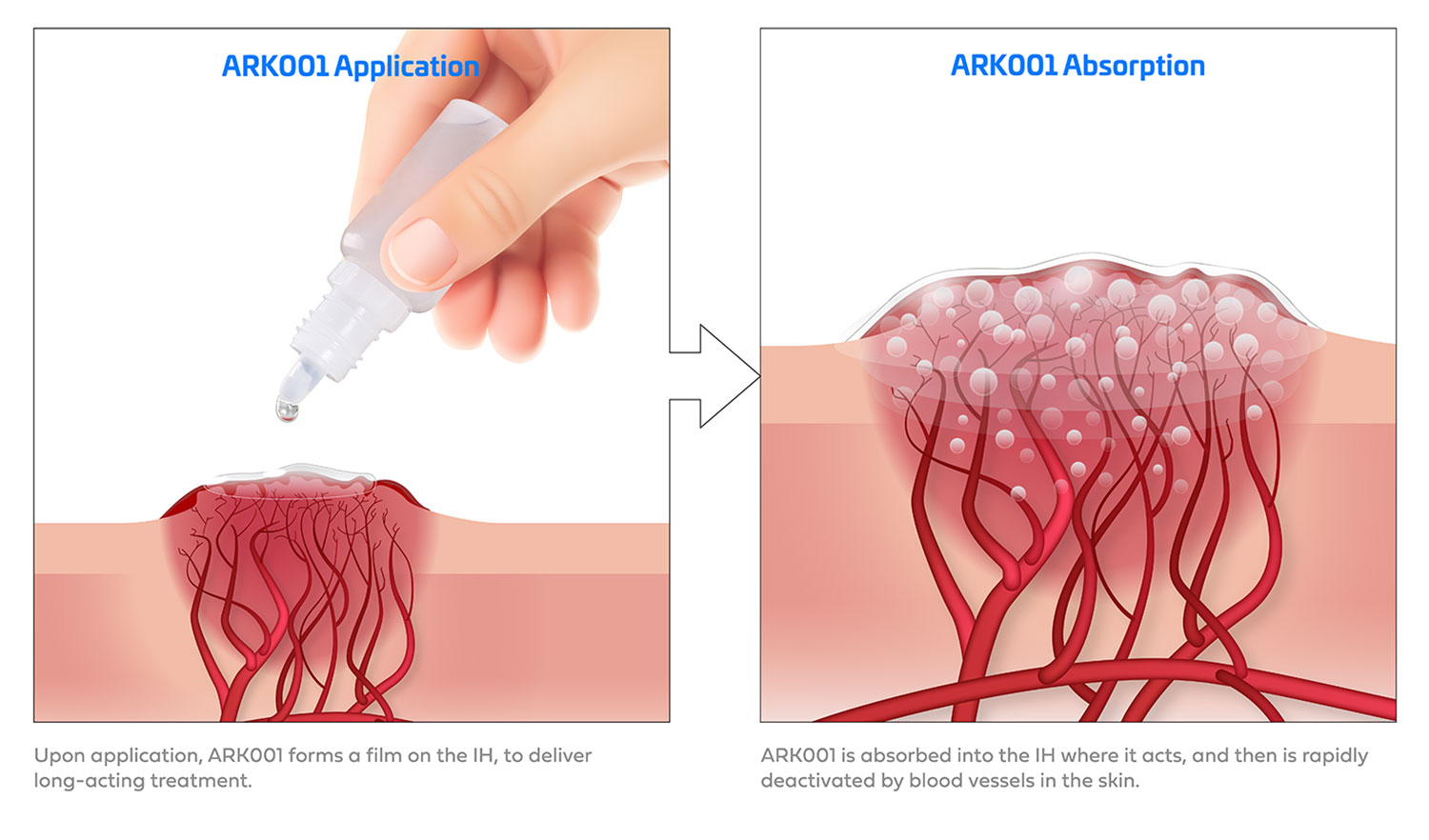
Fueled in part by a generous investment from UW Health Isthmus Project, ARK001 is still in early-stage development, although momentum for a safer alternative to existing treatments is clearly building.
Isthmus Project investment lends credibility
“This is a clever approach to an interesting problem,” says Elizabeth Hagerman, UW Health’s chief innovation officer and executive director of Isthmus Project. “Additionally, it’s a great fit for the Isthmus Project as we focus on supporting commercialization efforts of healthcare solutions developed by UW Health experts.”

Seth Reno, who joined Arkayli as president and CEO in 2021 after more than 30 years in the biopharma industry, was introduced to the project after meeting with Drolet and her two fellow Arkayli co-founders, Thom Rossi and Agis Kydonieus, both highly experienced drug development executives.
“This was clearly a strong team with a great idea,” Reno says. “Dr. Drolet sees hundreds of these patients each year and has been a key player in the evolution of infantile hemangioma treatment. This opportunity is exciting because we know that beta blockers are effective in treating IH. If we can more precisely treat babies with little to no systemic absorption of the beta blocker, and do it from the outside-in, rather than inside-out, the result would be a more effective treatment with minimal risk for systemic side-effects.”
Reno also credits Isthmus Project not only for its investment but its stamp of credibility.
“UW Health does not invest in projects on a whim,” Reno says. “They do extensive, thorough due diligence before making an investment. They’ve also provided added value by conducting their own market research which validated the need for such a product with pediatricians.”
Animal studies of ARK001 involving mini pigs, began in late 2022. Early toxicity studies are slated for 2023. Assuming these go smoothly, Arkayli’s next step would be seeking US Food and Drug Administration (FDA) for investigational new drug status, followed by clinical trials on infants with IH. “We’re not introducing a new molecular formulation,” says Drolet, “so our hope is that things will move forward at a quicker pace than if we were proposing a brand new drug. We’re hoping to reach the market by 2028.”